As we enter into the fall and winter of 2020, COVID-19 has consumed much of our everyday lives. From decisions on how we chose to spend our holidays to the reinstatement of stay-at-home advisories, it’s a position that none of us wanted to be in. However, recent reports from the pharmaceutical sector have provided us with some welcome news: effective vaccines are on the way! With this announcement comes a series of very legitimate questions and concerns from the general public:
-
What does this mean for the future?
-
How will the vaccine be distributed?
-
Will we be rid of this menace?
Before we can address these questions, we need to acknowledge the situation we’re in right in. The SARS-CoV-2 spread throughout the U.S. is at an all-time high, and there’s little reason to believe that caseloads will substantially decrease in the immediate future. Intensive care units (ICU for shorthand) are approaching capacity in many regions. Even in areas where ICU bed capacity has not maxed out yet, staff are already in full use. Many healthcare workers are experiencing severe burnout – both physically and emotionally – as they battle one surge after another. A hospital bed is useless without an accompanying healthcare provider, and they are approaching their limit. When I last wrote for SMERCONISH at the end of September, I outlined all the telltale signs of a massive fall-winter surge of COVID-19. Tragically, those signs came into being. We are certainly in for a challenging few months ahead.
On Monday, November 9th, Pfizer and BioNTech announced that their vaccine candidate BNT162b2 showed an efficacy of ~90% protection against SARS-CoV-2 relative to the placebo group. The Pfizer/BioNTech vaccine is an mRNA-based immunization platform and is given in a two-dose series. Granted, this was an interim report of the ongoing Phase-III trial of the BNT162b2 vaccine. The number of patients evaluated was not very high. One week later, Moderna similarly announced a preliminary assessment of their ongoing Phase-III trial with results in line with Pfizer/BioNTech. Both of these vaccines are cause for cautious optimism, especially as more interim evaluations are announced in the coming weeks and months. Even more so, the notion that a vaccine candidate has already shown promise in a Phase-III trial against a pathogen identified less than a year ago speaks volumes to the scientists and physicians working around the clock. In the field of infectious diseases, this turnaround time is practically unthinkable.
So what does this mean for the future? There are two parts to this question. First, we should consider the immediate future – the period between now and the public rollout of the first wave of vaccines. While our understanding of SARS-CoV-2 has evolved during the pandemic, the underlying guidance remains mostly unchanged:
-
Wear a mask.
-
Wash your hands.
-
Maintain physical distance from others, and
-
Move activities to well-ventilated areas.
If the general public followed these four recommendations consistently, caseloads would plummet. It is essential not to let our guard down even if good news is coming.
What does this mean for the future?
This question means delving into more intermediate-term vaccination goals, namely reducing the virus burden. This depends on the effectiveness of the vaccine and how much of the population can receive the vaccine. Pfizer/BioNTech and Moderna’sModerna’s two interim reports should give us confidence that vaccine efficacy will be high. Previously, Dr. Anthony Fauci commented that the aim for the first round of vaccines against SARS-CoV-2 should be > 70% effective. At the time, I thought that was reasonable, albeit high, expectation. This is a novel pathogen, after all. However, given the recent reports, there is every reason to believe that most of the vaccine candidates in Phase-III trials will confer high protection. Thus, it is of the utmost importance to encourage uptake of a vaccine as the higher the vaccine coverage in a community, the less virus burden there will be in the community. Also, by reducing the virus burden, contact tracing can become more effective. Testing turnaround times can be shortened as supplies will not be in such high demand.
In short, this is a huge deal in the intermediate-term goals for the future. By lowering the COVID-19 burden, we can begin to get this pandemic under control. Getting the vaccine candidates to the general public, though, might be tricky.
How will the vaccine be distributed?
Answering this question depends on the vaccine. For example, the Pfizer/BioNTech vaccine is based on mRNA technology. It has thus far used extreme cold temperature storage and shipping (-70°C or -94°F). Conversely, Moderna’sModerna’s mRNA-based vaccine can be stored in a typical freezer (-20°C or -4°F), or perhaps even at refrigeration temperatures (4°C or 39°F). The Janssen/Johnson and Johnson’sJohnson’s vaccine candidate, an adenovirus-based vaccine, is quite flexible since it can be stored at refrigeration temperatures. Similarly, Sanofi and Glaxosmith Kline’s recombinant vaccines can be stored at refrigeration temperatures. Given that there are different shipping and storage protocols for various vaccines, we must confront the reality that there will be logistical hurdles to a mass-scale rollout of vaccines.
Ultracold temperature freezers are relatively standard at academic institutions and healthcare facilities. They are routinely used for the preservation of cell lines, nucleic acids, and certain chemical compounds. As one would expect, ultracold freezers consume more energy than other units and can be expensive to operate. To give the reader an idea, Stanford University spent ~$5.6 million merely keeping them running on their campus in 2010. Energy-efficient models have since emerged, but unquestionably operating costs for these specialized freezers are high. Compounding this problem for Pfizer/BioNTech is that their vaccine is a two-dose series, meaning that every patient will require two immunizations several weeks apart. This is also true for the Moderna vaccine but does not require ultracold storage.
These logistical challenges will put a tremendous strain on on-site housing and administering vaccines requiring ultracold storage. This burden is likely to be disproportionately felt by rural communities, where the pandemic is currently raging, as was predicted by health experts. To administer vaccines requiring such storage, we will need to be steady and sound communication from the local level up to the federal level. Operation Warp Speed, the private-federal government partnership that began in the Spring of 2020, expressly set aside working teams to facilitate vaccines’ distribution when they have met safety and efficacy requirements.
We need to set reasonable expectations for the vaccine’s rollout and who should receive the first doses. The National Academies have convened a committee to recommend the first wave of SARS-CoV-2 vaccines’ ethical distribution. Considerations include likeliness of exposure, community health disparities, high-risk demographics, and geographic feasibilities. From there, the distribution of vaccines can progress to the general public, which I expect to occur in the Spring of 2021. I understand reading this can be frustrating, but the logistical hurdles to vaccinating hundreds of millions cannot be understated. This is true for vaccines requiring ultracold storage, as well as those requiring refrigeration temperatures.
Will we be rid of this menace?
The question on everyone’s mind. The answer ties directly into the first question, particularly in terms of long-term goals. The word “endemic” has been used increasingly by the scientific community to describe SARS-CoV-2 in the U.S. By this, we mean the virus has established a permanent residence within our country. Human-to-human transmission is sustained, and only through public health interventions such as vaccinations can we cease sustained transmission of the virus. A great comparison would be the measles virus, which was declared eliminated from the U.S. in the year 2000 by the World Health Organization. Are there still some sporadic outbreaks of measles in the U.S.? Yes. However, those outbreaks are self-limiting because much of the population has vaccine-acquired immunity towards the measles virus.
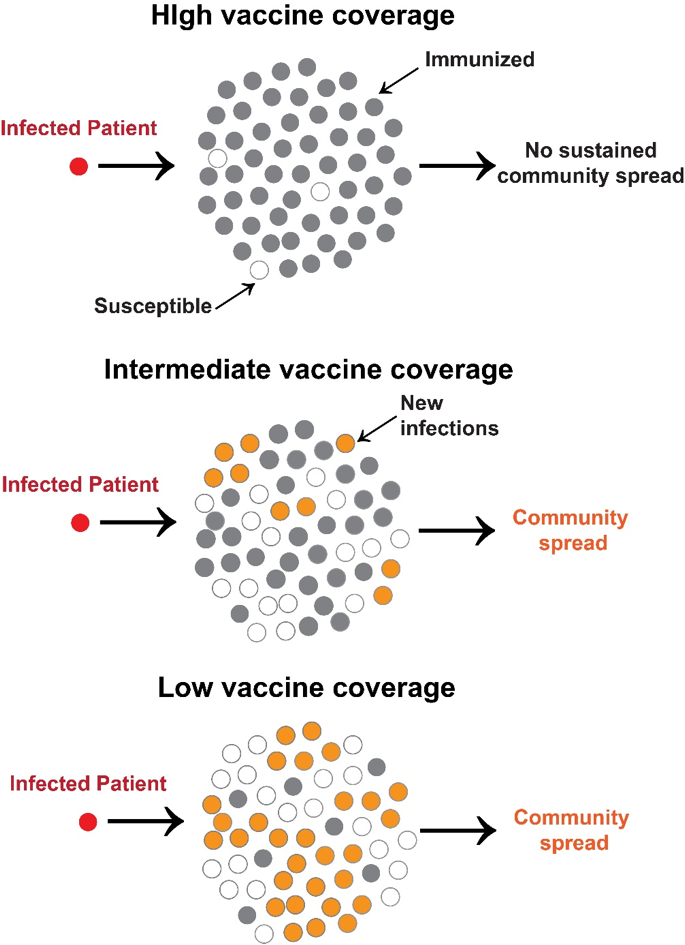
Take this illustration as an example, when an infected patient (red circle) comes into a population that has high vaccine coverage (gray circles), there is no community spread, even if there are a few susceptible patients within the population (white circles). This changes when vaccine coverage is lowered, and there is a higher percentage of susceptible individuals. In this case, subsequent transmission occurs (orange circles). As the percentage of susceptible individuals increases, so does the community spread. We achieved high vaccine coverage against measles; hence when the virus is introduced into a community, it cannot sustain transmission.
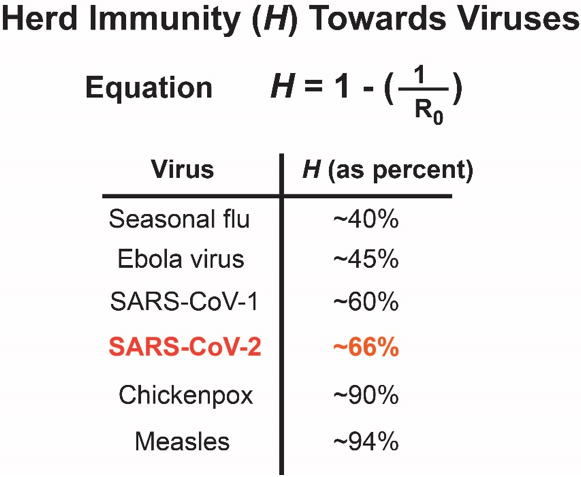
When a high enough percentage of the population has immunity towards a virus, there is nowhere for the virus to go. Its spread is halted. The term “herd immunity” is routinely used to address this scenario. Herd immunity is proportional to the basic reproductive number(R0) of a virus, or the number of new infections a single infection begets in a susceptible population. Some viruses, such as seasonal influenza, are low. In contrast, other viruses such as measles are incredibly high due to their exceptionally infectious nature. For SARS-CoV-2, the number is ~ 2.7.
I would stress to the audience that a country’s virus elimination through herd immunity has only ever been shown to occur through vaccination. We have precisely zero examples of a country eliminating a virus through infection-acquired herd immunity. This is even more true for viruses with high lethality rates since only those who survived can mount an effective immune response.
While preliminary data for vaccine effectiveness is promising, the reality is that we will be dealing with the menace of COVID-19 for the foreseeable future. It takes time for a population to achieve this vaccine-acquired herd immunity. There will inevitably be hiccups in vaccine distribution. I certainly hope that I’m wrong and that vaccine delivery, uptake, and efficacy are high, which would eliminate SARS-CoV-2 from the U.S. soon. However, again, we need to set reasonable expectations for how this will play out.
It is essential to take a step back and realize how quickly we’ve progressed in developing vaccine candidates against SARS-CoV-2. Delivery of these vaccines will be challenging. It will require infrastructure commitments that may not be immediately apparent to the general public. There will be bumps along the way, but I would urge patience. We’ve come a long way, and it is in everyone’s best interest to make the upcoming vaccine rollouts as safe, ethical, and efficient as possible.
______________________________________________________________________________________________________________________






